ST Pharm's article has been featured in the special edition RNA/Nucleic Acid of Nature Biopharma Dealmakers in November, 2023.
ST PHARM has established a cost-effective way to develop and produce messenger RNA (mRNA) therapeutics and vaccines. By investing in two technology platforms, the South Korea-based contract development and manufacturing organization (CDMO) has positioned itself to support external clients and its two biotech units without encountering intellectual property issues. The model enables ST PHARM both to meet rising demands for mRNA CDMO services and, through its biotechs, to support RNA-based drug and vaccine development partnerships.
Founded in 1983, ST PHARM is a leading gene-therapy CDMO with a history of supporting oligonucleotide projects. Management identified mRNA as a significant opportunity years before the pandemic validated the modality and set up a business focused on the oncology and autoimmune applications of the nucleic acid in 2018.
ST PHARM developed two mRNA platform technologies to facilitate its expansion. The development work has given the company 5′-capping analogs, marketed as SmartCap, and a lipid-nanoparticle (LNP) drug delivery system, STLNP. ST PHARM’s vision and willingness to invest in in-house platform technologies has enabled it not only to bypass intellectual property issues that hinder many CDMOs from entering the mRNA market but also to emerge as a leader in the space, as exemplified by its inclusion in South Korea’s mRNA vaccine consortium (Fig. 1).
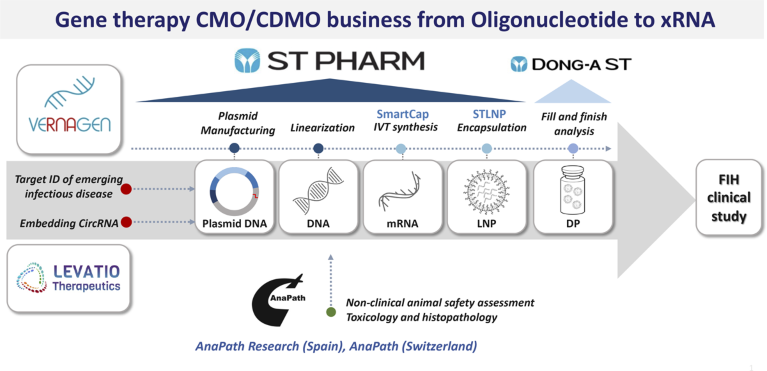
Fig. 1 | ST PHARM’s leading gene therapy CMO/CDMO business including two mRNA platform technologies, SmartCap and STLNP. CircRNA, circulating RNA; DP, drug product; FIH, first-in-human; IVT, in vitro transcription; LNP, lipid-nanoparticle; STLNP, lipid-nanoparticle drug delivery system.
A COVID-19 vaccine, based on ST PHARM’s technologies, against ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has completed a phase 1 clinical trial. An early-phase study of an ST PHARM pan-coronavirus vaccine, which could provide broad protection against COVID-19 variants and related viruses, is getting underway too.
Expanding into asset development
ST PHARM has leveraged its technologies to set up two biotechs in the US. One of the startups, Levatio Therapeutics, is working on circular RNA medicines. Circular RNA is a closed RNA without 5′ or 3′ ends, a unique structure that prevents degradation by exonucleases and makes the molecules more stable than the better-known linear mRNA.
San Diego-based Levatio has identified the emerging, next-generation circular-RNA modality as a way to develop cancer vaccines that target oncoviral antigens and neoantigens, chimeric antigen receptor (CAR) natural-killer T cells for use in cancer and autoimmune diseases, and treatments for infectious and degenerative diseases. The biotech’s pipeline features three drug candidates that modulate regulatory T cells to treat autoimmune diseases, and two immunotherapies that promote anticancer immunity.
Levatio’s links to ST PHARM are central to its ability to develop circular RNA candidates. While some other drug developers are shut out of the circular RNA space by their lack of platform technologies, Levatio is building on ST PHARM’s investment in the sector to unlock the inherent advantages of the modality. The platform makes Levatio a potential partner for companies that want to move into circular RNA.
ST PHARM’s other biotech, Vernagen, is an Atlanta-based developer of infectious-disease mRNA vaccines and antibody-encoding RNA candidates. Working with collaborators such as Emory University and the US Centers for Disease Control and Prevention, Vernagen has built a broad pipeline of candidates against global viral pathogens such as shingles virus and respiratory syncytial virus (RSV) as well as emerging, potential pandemic, and highly pathogenic viruses including Nipah virus, Chikungunya virus, yellow fever virus, and Heartland virus.
Both biotechs benefit from their relationship to ST PHARM. Possessing close, synergistic ties to a leading CDMO, the biotechs have access to technology platforms, integrated research and development (R&D) and production capabilities, and large-scale good manufacturing practice (GMP) capacity. Few biotechs or CDMOs possess mRNA capabilities, and, thus, Levatio and Vernagen’s ties to ST PHARM are a competitive advantage.
The pandemic validated ST PHARM’s capabilities. As COVID-19 disrupted other biopharma supply chains, ST PHARM, with its long history of manufacturing small molecules and oligonucleotides, used its internal capabilities to produce key starting materials and maintained output despite the effect of the pandemic on trade. That reliable supply chain now underpins Levatio and Vernagen.
Advancing the pipeline
Access to ST PHARM’s top-tier resources positions Levatio and Vernagen to quickly and cost-effectively advance candidates that address a range of major unmet medical needs. Levatio plans to move its two lead candidates, a neoantigen cancer vaccine and an anticancer immunostimulator, into investigational new drug (IND)-enabling studies in 2024 and to begin clinical trials the next year. Vernagen is also aiming to get multiple candidates ready for phase 1 clinical trials by 2025.
Biopharma companies that partner with Levatio or Vernagen to access platforms or assets, or outsource work to ST PHARM, will stand to benefit from the CDMO’s years of far-sighted investment in mRNA. Through this investment, ST PHARM has established technology platforms that underpin its biotechs, positioning the companies to support and accelerate their efforts in the development of life-changing mRNA therapeutics and vaccines.
To learn more